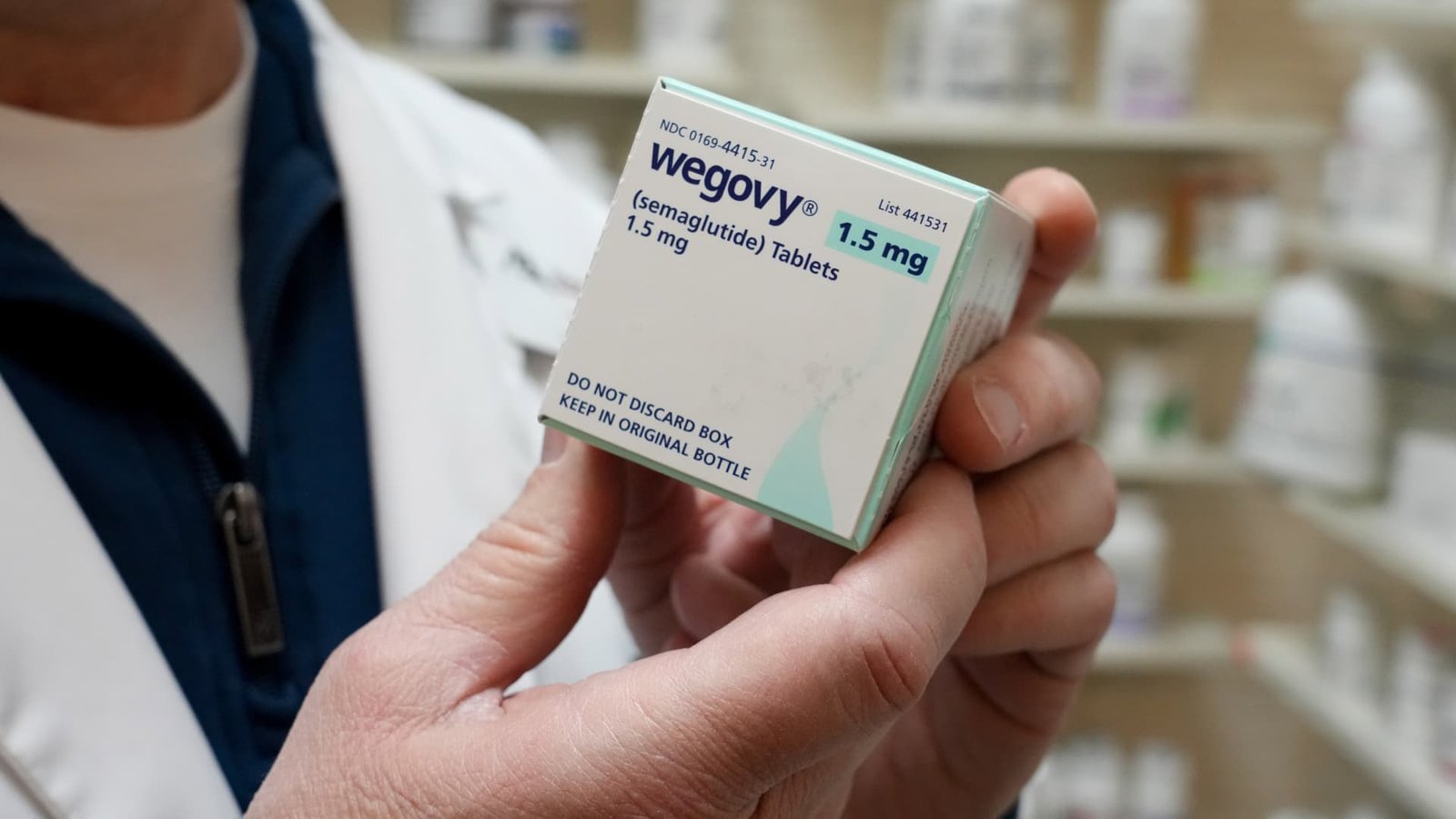
Shares of Novo Nordisk experienced an increase of over 8% following the initial prescription data for Wegovy, a new GLP-1 oral medication for obesity, released on January 5, 2026. Analysts from TD Cowen characterized the launch as a “solid start,” although they noted that one week’s data is insufficient to establish a trend.
Early metrics suggest strong demand for Wegovy, with 3,100 prescriptions reported in its first week according to IQVIA data, although some sources cite higher numbers. For instance, TD Cowen referenced data from Symphony indicating around 4,290 prescriptions were filled during the same period, primarily for the introductory dose. In comparison, Eli Lilly’s injectable obesity treatment, Zepbound, recorded about 1,900 prescriptions during its launch week.
As both companies vie for market share in the expanding obesity and diabetes drug category, Eli Lilly, having captured a significant portion of the market in 2025, is preparing to launch its own oral treatment, orforglipron. Analysts believe the introduction of this new drug could shift demand dynamics. Novo Nordisk’s Wegovy, classified as a peptide medication, comes with dietary restrictions—users must avoid food or drink for 30 minutes post-consumption—which may influence user adoption compared to Eli Lilly’s small-molecule drug, which lacks such constraints.
The coming weeks will provide a clearer picture of Wegovy’s market performance, particularly in the direct-to-consumer sector, which analysts believe has substantial potential.
Why this story matters:
- The competition between Novo Nordisk and Eli Lilly could significantly impact the obesity drug market and patient access to new treatment options.
Key takeaway:
- Wegovy’s initial success highlights strong demand, but the competitive landscape may shift with the upcoming launch of Eli Lilly’s oral medication.
Opposing viewpoint:
- The dietary restrictions associated with Wegovy may deter potential users compared to Eli Lilly’s more flexible oral option, potentially limiting its uptake.